Ionization
As penetrating radiation moves from point to point in matter, it loses its energy through various interactions with the atoms it encounters. The rate at which this energy loss occurs depends upon the type and energy of the radiation and the density and atomic composition of the matter through which it is passing.
The various types of penetrating radiation impart their energy to matter primarily through excitation and ionization of orbital electrons. The term "excitation" is used to describe an interaction where electrons acquire energy from a passing charged particle but are not removed completely from their atom. Excited electrons may subsequently emit energy in the form of x-rays during the process of returning to a lower energy state. The term "ionization" refers to the complete removal of an electron from an atom following the transfer of energy from a passing charged particle. In describing the intensity of ionization, the term "specific ionization" is often used. This is defined as the number of ion pairs formed per unit path length for a given type of radiation.
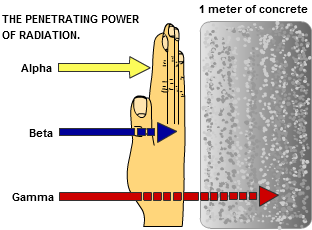 Because of their double charge and relatively slow velocity, alpha particles have a high specific ionization and a relatively short range in matter (a few centimeters in air and only fractions of a millimeter in tissue). Beta particles have a much lower specific ionization than alpha particles and, generally, a greater range. For example, the relatively energetic beta particles from P32 have a maximum range of seven meters in air and eight millimeters in tissue. The low energy betas from H3, on the other hand, are stopped by only six millimeters of air or six micrometers of tissue.
Because of their double charge and relatively slow velocity, alpha particles have a high specific ionization and a relatively short range in matter (a few centimeters in air and only fractions of a millimeter in tissue). Beta particles have a much lower specific ionization than alpha particles and, generally, a greater range. For example, the relatively energetic beta particles from P32 have a maximum range of seven meters in air and eight millimeters in tissue. The low energy betas from H3, on the other hand, are stopped by only six millimeters of air or six micrometers of tissue.
Gamma-rays, x-rays, and neutrons are referred to as indirectly ionizing radiation since, having no charge, they do not directly apply impulses to orbital electrons as do alpha and beta particles. Electromagnetic radiation proceeds through matter until there is a chance of interaction with a particle. If the particle is an electron, it may receive enough energy to be ionized, whereupon it causes further ionization by direct interactions with other electrons. As a result, indirectly ionizing radiation (e.g. gamma, x-rays, and neutrons) can cause the liberation of directly ionizing particles (electrons) deep inside a medium. Because these neutral radiations undergo only chance encounters with matter, they do not have finite ranges, but rather are attenuated in an exponential manner. In other words, a given gamma ray has a definite probability of passing through any medium of any depth.
Neutrons lose energy in matter by collisions which transfer kinetic energy. This process is called moderation and is most effective if the matter the neutrons collide with has about the same mass as the neutron. Once slowed down to the same average energy as the matter being interacted with (thermal energies), the neutrons have a much greater chance of interacting with a nucleus. Such interactions can result in material becoming radioactive or can cause radiation to be given off.
The Three Mechanisms of Ionization:
The amount of ionization that occurs is dependent upon two principal factors, (1) the radiation energy, and (2) the type of material for which the radiation is interacting. For a given material, the level of ionization will vary with varying levels of radiation energy. There are three principal mechanisms of ionization that are of interest in radiography. These include the Photoelectric effect, Compton effect, and Pair Production.
First Principal Mechanism of Ionization
The photoelectric effect of ionization involves the complete absorption of the photon energy during the process of knocking an electron out of orbit. This process primarily occurs with low energy photons ranging in energy between 10 Kev and less than 500 Kev. During this process, when the photon liberates the electron, all of the photon's energy is transferred to create the ion pair and total absorption has occurred. Remember, there is a binding force that the holds the electron in its orbital shell. The amount of energy required to create the ion pair must be at least equal to this binding force.
If during the ionization process, only part of the photons energy is needed to liberate the electron, the rest of the energy is transferred to the electron in the form of speed (velocity). Now that all of the photon's energy is accounted for, the photon ceases to exist and total absorption has occurred. Remember that a photon is not a particle, but acts like one. When the energy of the photon is used, there is nothing left to cause further ionization. Keep in mind that electrons orbit in various shells of the atom and not all electrons have the same binding energy. This binding energy is dependent upon the elements (Z) number and the position of the electron in the atom. Those electrons nearer the nucleus possess greater binding energy and will require greater photon energy to remove them than will electrons in the outer shells.
Second Principal Mechanism of Ionization
The second type of ionization is known as the Compton effect (sometimes referred to as Compton Scatter). In this form of interaction, the initial photon energy is higher than that of the Photoelectric effect. The primary difference is that not all of the photon energy will be utilized in liberating and accelerating an electron. There is also energy left over to cause further ionization.
The Compton effect may occur when photon energies range from approximately 50 Kev to 3 Mev. Notice that Compton effect overlaps that of the Photoelectric effect. At relatively low energies, the Photoelectric effect is the dominant form of interaction, and it becomes less predominant as energy levels increase. It has been determined that the Compton effect starts slowly and becomes more dominant at energies above 100-150 Kev.
In the Compton effect process of ionization, not all of the photon energy is absorbed during the liberation of the electron. This excess energy takes on the form of a new photon having longer wavelength (less energy) than that of the original photon. In addition, the new photon moves through the material in a new path. This is where the term scatter derives from.
So what happens with this new photon?
The new photon will continue to interact with the material and its energy may be absorbed in the same manner as the original photon. The photon may continue to go through several Compton effect actions depending on its original energy, and eventually it will go through the Photoelectric effect as the energy diminishes.
It should be noted, that the change in the direction of the new photon due to Compton effect is dependent on the energy of the photon. The higher the energy of the photon, the smaller the change in direction resulting from ionization. Keep in mind that after Compton effect, the path of the resulting photon is never the same as the original. Relatively low energy photons may result in a direction that is completely opposite the original direction.
Third Principal Mechanism of Ionization
The third process of ionization is known as pair-production. In this process, the initial photon energy is very high, normally occurring at energies of 1.02 Mev and above. This particular process does not involve orbital electrons, rather the interaction occurs near the nucleus of the atom instead.
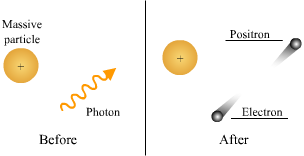
As the photon energy approaches the nucleus of the atom, it is changed into an electron -positron pair. The electron and positron move in different paths away from each other. A positron is nuclear in origin, possessing a positive charge, and mass equal to that of an electron. Technically a positron is the sister particle to the electron. Being positively charged, the positron immediately joins with an electron. The result of this process is annihilation of the positron, and the emission of two new photons, each with equal energy, but one half that of the original photons. These two new photons continue to go through ionization, eventually producing the Compton effect, and finally diminishing to the Photoelectric effect and total absorption.
Subionization
In addition to understanding the processes of ionization, we need to consider the liberated electrons. What about the free electrons that have been liberated during the process? Every method of ionization results in some form of electron liberation. These electrons possess energy (Kinetic energy), or motion. Where do they go?
Eventually the energy of the electron must be absorbed as well. The energy of a moving electron can be absorbed in different ways. The electron may collide with another orbital electron and knock it out. Resulting in a loss of energy, or sharing of energy due to liberating another electron. If the electrons energy transfer is not totally absorbed, it may continue to liberate other electrons. Liberated electrons may also have enough sufficient energy to continue liberating other electrons. This process may continue until minimal energy remains in any one electron. These low energy electrons will eventually interact with an atom in what is known as Subionization. Atoms are not ionized by this process. Rather, the orbital electrons are given a little excess energy, which will be given off eventually in the form of low energy electromagnetic radiation. This electromagnetic form may be ultraviolet light, visible light, or heat energy. It should be noted that although all X- and gamma ray absorption eventually ends up this way, the actual quantities are very small in relation to the mass of the material for which the interaction is occurring. The actual effects would be extremely small and unnoticeable, unless we had some sort of instrumentation that was highly sensitive.