Molecules and Compounds
After reading this section you will be able to do the following:
- Explain the difference between molecules and compounds.
- Name some common molecules.
Molecules
A molecule is a collection of atoms that are bonded together. These atoms can either be of the same element or of different elements. When the molecule is made of different elements is can also be called a compound. Based on these definitions, all compounds are molecules but not all molecules are compounds.
When a molecule is made of only two atoms it is called a diatomic molecule. There are 7 gases that form stable diatomic molecules near room temperature: hydrogen, nitrogen, oxygen, fluorine, chlorine, bromine, and iodine. This means that the nitrogen and oxygen in Earth's atmosphere are actually diatomic molecules of oxygen and nitrogen. The diatomic nature of hydrogen and oxygen is depicted in the figure below: in each gas cylinder the gas is composed of diatomic molecules which are shown as two spheres (atoms) connected by a bar that represents the bond between them.
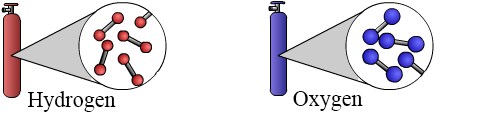
Compounds
As previously mentioned, compounds are molecules that consist of at least two different types of elements. Examples of compounds include water, carbon dioxide, and ammonia. When compounds contain at least one atom of carbon bonded to other elements (commonly hydrogen, oxygen, or nitrogen) then the compound is called an organic compound.
Review:
- Molecules are collections of atoms bonded together.
- When molecules consist of two or more different types of atoms they are called compounds.