Why Things Fluoresce
Fluorescent penetrant materials usually contain several dye compounds that are especially suited for the production of fluorescence. Fluorescence is the process wherein a molecule absorbs a photon of radiant energy at a particular wavelength and then quickly re-emits the energy at a slightly longer wavelength. It is the rapid and short-term re-emittance of energy that distinguishes fluorescence from phosphorescence. Phosphorescence is usually the result of a chemical reaction which sustains the release of energy for a significant period of time. Fluorescence was first described in the sixteenth century and was probably observed long before that time since a large number of plant and animal products fluoresce.
The phenomenon of fluorescence requires a short lesson in quantum mechanics which explains why fluorescence was not understood until the twentieth century. In the nineteenth century, Huygen's wave theory of light had replaced Newton's concept of the particulate nature of light and fluorescence was one of the embarrassing phenomena which simply could not be explained by use of the wave theory. The wave theory, as with most classical physics, generally assumes change to be a continuous process with no abrupt changes. Near the beginning of the twentieth century, Max Planck suggested that energy changes might occur in a stepwise manner. This concept forms the basis of quantum mechanics and Einstein applied the quantum concept of energy to light and revived the idea of the particulate nature of light. Planck formalized the relationship with the equation shown below:
Where:
E = energy
h = a constant
ν = the frequency of light
 This equations shows that the size of the energy steps change with the frequency or wavelength of the light. Einstein introduced the term photon to describe the smallest increments of light.
This equations shows that the size of the energy steps change with the frequency or wavelength of the light. Einstein introduced the term photon to describe the smallest increments of light.
In today's current model of the atom, protons and neutrons are found in the nucleus and electrons are found spinning around outside the nucleus. Electrons spin and rotate around the nucleus billions of times a second. According to modern theory, electrons are arranged in energy levels as they rotate around the nucleus. When electrons gain or lose energy, they jump between energy levels as they are rotating around the nucleus. As electrons gain energy, they move to the third, or outer level and as they lose energy, they move to the inner or first energy level. Since the energy of the system is restricted to certain energy values, the atom is said to be quantized. In the animated image below, it can be seen that the electrons move to a different energy state only when a specific amount of energy is added to or removed from the system.
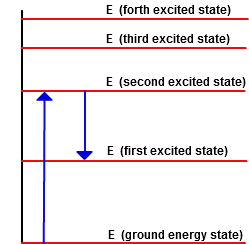
Another way of illustrating this point is with an energy diagram as presented below. This diagram shows the quantized energy levels for an atom. Each energy level corresponds to a quantum state of the atom. The lowest energy state is called the ground state and is the E0 line in the diagram. If energy is added to the system, an electron or electrons will jump to a higher level and the atom is said to be at an excited state. The upward arrow in the illustration represents a quantum jump of the atom from the ground state to the second excited state. Depending on the amount of energy input into the atom, the energy jump could have been to any of the levels. However, the jump must be to one of the levels shown, as the atom cannot have an intermediate value of energy. Atoms will generally be in their ground state.
 When considering fluorescence, energy must be considered at a molecular level. When molecules form, two or more atoms form an association where the energy of the molecule is lower than that of the constituent atoms when they were separate. The total energy of the molecule is the sum of the energies holding the nuclei together and the energy of the chemical bonds holding the molecule together. Molecules have rotational, vibrational and electronic (due to the electrons) energy. It is the vibrational and electronic energies of the molecule that contribute to fluorescence. Molecules, like atoms, will generally be in their ground state. Molecules can move to a greater energy state only when energy is added to their system. One of the ways a molecule can gain energy is by absorbing light. If a molecule absorbs light, the energy of the light must be equal to the energy required to put the molecule in one of the higher energy states. When a molecule reaches an excited state, it does not stay there for very long. Rather it quickly returns to a lower energy state either by emitting light or colliding with another atomic particle. When a molecule emits light, the energy of that light is equal to the energy difference between the quantum levels that molecules has moved between.
When considering fluorescence, energy must be considered at a molecular level. When molecules form, two or more atoms form an association where the energy of the molecule is lower than that of the constituent atoms when they were separate. The total energy of the molecule is the sum of the energies holding the nuclei together and the energy of the chemical bonds holding the molecule together. Molecules have rotational, vibrational and electronic (due to the electrons) energy. It is the vibrational and electronic energies of the molecule that contribute to fluorescence. Molecules, like atoms, will generally be in their ground state. Molecules can move to a greater energy state only when energy is added to their system. One of the ways a molecule can gain energy is by absorbing light. If a molecule absorbs light, the energy of the light must be equal to the energy required to put the molecule in one of the higher energy states. When a molecule reaches an excited state, it does not stay there for very long. Rather it quickly returns to a lower energy state either by emitting light or colliding with another atomic particle. When a molecule emits light, the energy of that light is equal to the energy difference between the quantum levels that molecules has moved between.